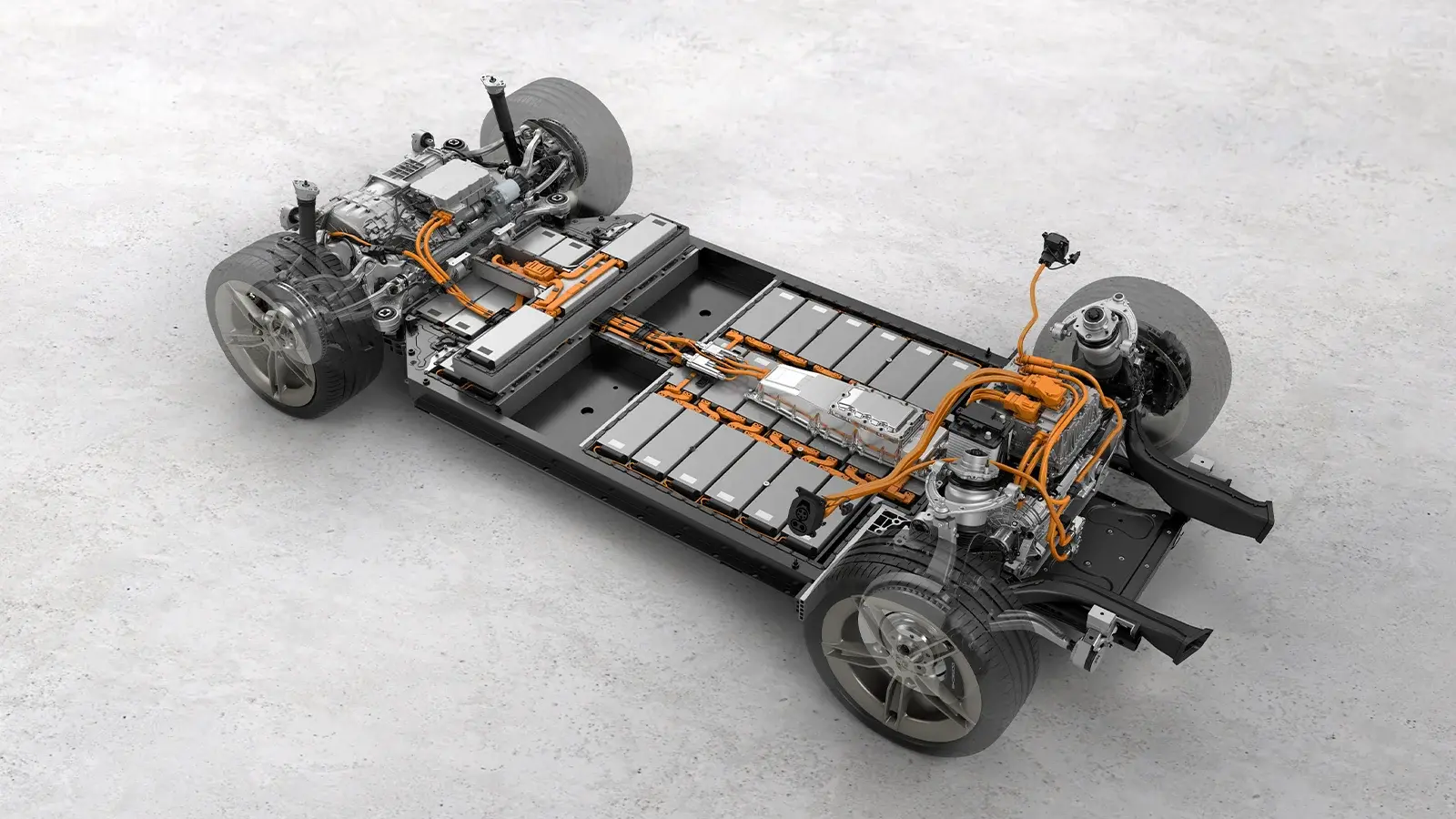
How EV Batteries Function
Electric vehicles (EVs) rely on advanced battery systems to power the motor, electronics, and other systems. But what exactly happens inside the battery when you press the accelerator? This guide explains how EV batteries function and what makes them so efficient.
The Basics of Energy Storage
EV batteries store energy in chemical form and release it as electricity when needed. Each battery contains hundreds or thousands of small cells. These cells are organized into modules, which are then combined into a battery pack.
The battery pack stores electricity in the form of direct current (DC). During driving, the vehicle’s inverter changes this to alternating current (AC) to supply the electric motor. In some EV designs, the DC current powers the motor directly.
Inside Each Battery Cell
Each lithium-ion battery cell includes:
- Cathode (positive electrode): Made from materials like lithium iron phosphate or nickel manganese cobalt
- Anode (negative electrode): Typically made from graphite
- Electrolyte: A fluid that allows lithium ions to flow between electrodes
- Separator: A layer that prevents the electrodes from touching
When charging, lithium ions move from the cathode to the anode. When the car is driving, the ions flow back toward the cathode, creating an electrical current that powers the vehicle.
What the Battery Management System Does
The battery management system (BMS) monitors and regulates the battery to maintain safe and efficient operation. Its functions include:
- Controlling voltage and current during charging and discharging
- Preventing overheating or overcharging
- Balancing the power across battery cells
- Extending battery life and preserving safety
A well-designed BMS is critical to the performance and lifespan of an EV battery.
Power vs. Energy: What’s the Difference?
- Power (kilowatts or kW) determines how quickly the battery can deliver energy. Higher power means faster acceleration.
- Energy (kilowatt-hours or kWh) refers to the total amount of energy the battery can store. More stored energy means longer range.
You can think of it like a water tank. Energy is the size of the tank, while power is the speed at which water flows from it.
Regenerative Braking: Capturing Energy While Slowing Down
Regenerative braking allows an EV to recover energy during deceleration. When you lift your foot off the accelerator or apply the brake, the electric motor acts as a generator and sends some of that energy back to the battery. This process improves overall efficiency, especially in city driving.

How Batteries Stay at the Right Temperature
Batteries perform best within a moderate temperature range. EVs use thermal management systems to maintain the right operating conditions.
- In hot weather, cooling systems prevent the battery from overheating.
- In cold climates, built-in heaters warm the battery to support performance and allow regenerative braking to work effectively.
Controlling battery temperature helps extend its lifespan and maintain a consistent range.
How Long Charging Takes
Charging speed depends on several factors:
- The battery’s total capacity
- The level of the charger being used (Level 1, Level 2, or DC Fast Charging)
- The battery’s current state of charge
- Ambient temperature
For example, a Level 2 home charger typically adds 20 to 30 miles of range per hour. A DC fast charger can bring the battery to 80 percent in 20 to 40 minutes, depending on the vehicle.
Final Thoughts
EV batteries are complex systems, but they are built for long life, efficiency, and safe operation. By understanding how they function, from cell chemistry to power delivery, you can get the most from your electric vehicle.
Keep Exploring EV Battery Essentials
Exploring EV battery fundamentals:
← Go Back: Introduction to EV batteries
Discover Next: Different Types of EV Batteries →